Place the Steps in the Correct Order to Review the Process of Enzyme Repression
Introduction
The discovery of the get-go microRNA (miRNA), lin-4, in 1993 by the Ambros and Ruvkun groups in Caenorhabditis elegans (ane, 2) has revolutionized the field of molecular biology. Years before, lin-four was characterized by the Horvitz's lab as ane of the genes that regulate temporal development of C. elegans larvae (3, 4). Later in 1987, the same group institute that a mutation in lin-4 had an opposite phenotype to a mutation in another factor, lin-xiv, yet a lin-xiv suppressor mutation in a naught-lin-4 line was wildtype (5, half dozen). Both Ambros and Ruvkun continued to study lin-4 and lin-14 afterwards leaving the Horvitz's lab, only to observe subsequently that lin-iv was not a protein-coding RNA simply indeed a small-scale not-coding RNA (vii, 8). They also establish that lin-fourteen was post-transcriptionally downregulated through its 3′ untranslated region (UTR) and that lin-4 had a complementary sequence to that of the three′ UTR of lin-14 (one). Therefore, they proposed that lin-4 regulates lin-fourteen at the post-transcriptional level (2). Since and then, miRNAs have been detected in all animal model systems and some were shown to be highly conserved across species (9–12). New miRNAs are still being discovered (thirteen) and their roles in gene regulation are well recognized.
miRNAs are small non-coding RNAs, with an average 22 nucleotides in length. Most miRNAs are transcribed from Deoxyribonucleic acid sequences into primary miRNAs (pri-miRNAs) and processed into forerunner miRNAs (pre-miRNAs) and mature miRNAs. In nearly cases, miRNAs collaborate with the iii′ UTR of target mRNAs to suppress expression (14). However, interaction of miRNAs with other regions, including the v′ UTR, coding sequence, and cistron promoters, accept likewise been reported (15). Furthermore, miRNAs have been shown to activate gene expression under certain conditions (sixteen). Recent studies have suggested that miRNAs are shuttled between different subcellular compartments to control the rate of translation, and fifty-fifty transcription (17).
miRNAs are critical for normal animal development and are involved in a diversity of biological processes (eighteen). Aberrant expression of miRNAs is associated with many human diseases (19, 20). In addition, miRNAs are secreted into extracellular fluids. Extracellular miRNAs have been widely reported as potential biomarkers for a variety of diseases and they as well serve as signaling molecules to mediate cell-cell communications (21–23). In this review, we have provided a cursory overview of the different pathways of miRNA biogenesis in animals and the expanding complication of their regulation of gene expression. Moreover, we have discussed the dynamics of miRNA intracellular localization and role. Finally, we accept summarized the secretion and circulation of miRNAs and the potential roles of extracellular miRNAs in mediating intercellular communications.
Biogenesis of miRNAs
miRNA biogenesis starts with the processing of RNA polymerase 2/Iii transcripts postal service- or co-transcriptionally (14). Nearly half of all currently identified miRNAs are intragenic and processed mostly from introns and relatively few exons of protein coding genes, while the remaining are intergenic, transcribed independently of a host cistron and regulated by their own promoters (xiii, 24). Sometimes miRNAs are transcribed equally one long transcript called clusters, which may have similar seed regions, and in which example they are considered a family (25). The biogenesis of miRNA is classified into canonical and non-approved pathways (Figure i).
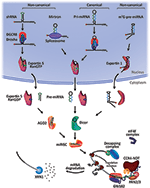
Figure one. MicroRNA biogenesis and machinery of action. Canonical miRNA biogenesis begins with the generation of the pri-miRNA transcript. The microprocessor complex, comprised of Drosha and DiGeorge Syndrome Critical Region 8 (DGCR8), cleaves the pri-miRNA to produce the precursor-miRNA (pre-miRNA). The pre-miRNA is exported to the cytoplasm in an Exportin5/RanGTP-dependent manner and processed to produce the mature miRNA duplex. Finally, either the 5p or 3p strands of the mature miRNA duplex is loaded into the Argonaute (Agone) family of proteins to course a miRNA-induced silencing complex (miRISC). In the non-approved pathways, small hairpin RNA (shRNA) are initially cleaved by the microprocessor circuitous and exported to the cytoplasm via Exportin5/RanGTP. They are further processed via AGO2-dependent, but Dicer-independent, cleavage. Mirtrons and 7-methylguanine capped (msevenOne thousand)-pre-miRNA are dependent on Dicer to complete their cytoplasmic maturation, simply they differ in their nucleocytoplasmic shuttling. Mirtrons are exported via Exportin5/RanGTP while chiliadsevenG-pre-miRNA are exported via Exportin1. All pathways ultimately lead to a functional miRISC complex. In most cases, miRISC binds to target mRNAs to induce translational inhibition, about likely by interfering with the eIF4F complex. Adjacent, GW182 family proteins bound to Argonaute recruit the poly(A)-deadenylases PAN2/3 and CCR4-NOT. PAN2/3 initiates deadenylation while the CCR4-Not complex completes the process, leading to removal of the m71000 cap on target mRNA past the decapping complex. Decapped mRNA may then undergo 5′−iii′ degradation via the exoribonuclease XRN1. Modified from Hayder et al. (26).
The Canonical Pathway of miRNA Biogenesis
The canonical biogenesis pathway is the dominant pathway by which miRNAs are processed. In this pathway, pri-miRNAs are transcribed from their genes and and so processed into pre-miRNAs by the microprocessor complex, consisting of an RNA bounden protein DiGeorge Syndrome Disquisitional Region 8 (DGCR8) and a ribonuclease 3 enzyme, Drosha (27). DGCR8 recognizes an N6-methyladenylated GGAC and other motifs inside the pri-miRNA (28), while Drosha cleaves the pri-miRNA duplex at the base of operations of the characteristic hairpin structure of pri-miRNA. This results in the germination of a ii nt three′ overhang on pre-miRNA (29). Once pre-miRNAs are generated, they are exported to the cytoplasm by an exportin v (XPO5)/RanGTP complex so processed by the RNase Iii endonuclease Dicer (27, 30). This processing involves the removal of the terminal loop, resulting in a mature miRNA duplex (31). The directionality of the miRNA strand determines the name of the mature miRNA form. The 5p strand arises from the v′ end of the pre-miRNA hairpin while the 3p strand originates from the iii′ end. Both strands derived from the mature miRNA duplex can exist loaded into the Argonaute (Ago) family of proteins (AGO1-4 in humans) in an ATP-dependent manner (32). For any given miRNA, the proportion of Agone-loaded 5p or 3p strand varies greatly depending on the cell blazon or cellular environment, ranging from near equal proportions to predominantly ane or the other (33). The selection of the 5p or 3p strand is based in function on the thermodynamic stability at the five′ ends of the miRNA duplex or a 5′ U at nucleotide position ane (34). Generally, the strand with lower v′ stability or 5′ uracil is preferentially loaded into Agone, and is deemed the guide strand. The unloaded strand is called the passenger strand, which will be unwound from the guide strand through various mechanisms based on the degree of complementarity. The passenger strands of miRNA that contain no mismatches are cleaved by AGO2 and degraded by cellular machinery which can produce a strong strand bias. Otherwise, miRNA duplexes with central mismatches or non-AGO2 loaded miRNA are passively unwound and degraded (14).
Non-canonical miRNA Biogenesis Pathways
To date, multiple non-canonical miRNA biogenesis pathways have been elucidated (Figure i). These pathways make use of different combinations of the proteins involved in the approved pathway, mainly Drosha, Dicer, exportin five, and AGO2. In general, the non-canonical miRNA biogenesis can be grouped into Drosha/DGCR8-contained and Dicer-independent pathways. Pre-miRNAs produced by the Drosha/DGCR8-contained pathway resemble Dicer substrates. An instance of such pre-miRNAs is mirtrons, which are produced from the introns of mRNA during splicing (35, 36). Another example is the seven-methylguanosine (10007G)-capped pre-miRNA. These nascent RNAs are straight exported to the cytoplasm through exportin one without the need for Drosha cleavage. There is a potent 3p strand bias most likely due to the grand7G cap preventing 5p strand loading into Argonaute (37). On the other hand, Dicer-independent miRNAs are processed by Drosha from endogenous short hairpin RNA (shRNA) transcripts (38). These pre-miRNAs require AGO2 to consummate their maturation within the cytoplasm because they are of insufficient length to be Dicer-substrates (38). This in turn promotes loading of the entire pre-miRNA into AGO2 and AGO2-dependent slicing of the 3p strand. The 3′-five′ trimming of the 5p strand completes their maturation (39).
Mechanisms of miRNA-Mediated Gene Regulation
Most studies to date have shown that miRNAs bind to a specific sequence at the 3′ UTR of their target mRNAs to induce translational repression and mRNA deadenylation and decapping (forty, 41). miRNA binding sites have also been detected in other mRNA regions including the 5′ UTR and coding sequence, as well every bit inside promoter regions (42). The binding of miRNAs to v′ UTR and coding regions have silencing effects on gene expression (43, 44) while miRNA interaction with promoter region has been reported to induce transcription (45). However, more studies are required to fully understand the functional significance of such fashion of interaction.
MicroRNA-Mediated Cistron Silencing via miRISC
The minimal miRNA-induced silencing circuitous (miRISC) consists of the guide strand and AGO (46). The target specificity of miRISC is due to its interaction with complementary sequences on target mRNA, called miRNA response elements (MREs). The degree of MRE complementarity determines whether there is AGO2-dependent slicing of target mRNA or miRISC-mediated translational inhibition and target mRNA decay (47). A fully complementary miRNA:MRE interaction induces AGO2 endonuclease activity and targets mRNA cleavage (47). However, this interaction destabilizes the association between Ago and the 3′ stop of the miRNA promoting its degradation (48, 49).
In animal cells, the majority of miRNA:MRE interactions are not fully complementary (50). About MREs incorporate at least cardinal mismatches to their guide miRNA, preventing AGO2 endonuclease action. Consequently, AGO2 acts equally a mediator of RNA interference, similar to the not-endonucleolytic AGO family members (AGO1, 3, and four in humans). In many cases, a functional miRNA:MRE interaction occurs via the v' seed region (nucleotides 2–eight) (42, 51). Even so, boosted paring at the three′ end aids in the stability and specificity of the miRNA-target interaction (15).
The formation of a silencing miRISC complex starts with the recruitment of the GW182 family of proteins by miRISC; GW182 provides the scaffolding needed to recruit other effector proteins (52), such as the poly(A)-deadenylase complexes PAN2-PAN3 and CCR4-Non, following miRNA:target mRNA interaction (50, 53). Target mRNA poly(A)-deadenylation is initiated by PAN2/iii and completed by the CCR4-NOT complex. The interaction between the tryptophan (W)-repeats of GW182 and poly(A)-binding poly peptide C (PABPC) promotes efficient deadenylation (50). Subsequently, decapping takes place facilitated by decapping protein two (DCP2) and associated proteins (52), followed past v′−3′ deposition by exoribonuclease 1 (XRN1) (54) (Figure i).
MicroRNA-Mediated Translational Activation
Although nigh studies are focused on how miRNAs inhibit gene expression, some have also reported upward-regulation of factor expression by miRNAs. In serum starved cells, AGO2 and another protein related to the miRNA-protein complex (microRNPs), Fragile-x-mental retardation related protein 1 (FXR1), were associated with AU-rich elements (AREs) at 3′ UTR to activate translation (55). Several miRNAs, including let-vii, were found to be associated with AGO2 and FXR1 to activate translation during jail cell wheel arrest, simply they inhibit translation in proliferating cells (55). Upregulation of gene expression past miRNAs was also observed in quiescent cells, such as oocytes (56, 57). The miRNA-mediated activation of translation involves AGO2 and FXR1 instead of GW182 (56). Other examples of gene activation past miRNAs include binding to the 5′ UTR of mRNAs encoding ribosomal proteins during amino acid starvation (58); thus suggesting that miRNA-mediated upregulation of gene expression occurs under specific conditions.
MicroRNA-Mediated Transcriptional and Mail service-transcriptional Factor Regulation Within the Nucleus
Through Importin-eight or Exportin-1, human AGO2 shuttles betwixt the nucleus and cytoplasm via its interaction with TNRC6A (a GW182 family unit protein) which contains a nuclear localization and export signal (59). Nuclear localized miRISC was found to regulate both transcriptional rates and post-transcriptional levels of mRNA (59–61) and associate with euchromatin at gene loci with active transcription (62). Withal, our understanding of when and how miRNAs exert their functions in the nucleus is still limited.
It has been reported that low molecular weight miRISC tin can interact with mRNAs inside the nucleus and induce nuclear mRNA deposition, although the mechanism behind this is unclear (59, 61, 63). Enrichment of miRNA at actively transcribed genes may advise that miRISC interacts with target mRNA co-/post-transcriptionally. The involvement of AGO and Drosha in mRNA splicing (64, 65) farther supports co-transcriptional miRISC:mRNA interactions. miRISC may also regulate transcription directly. A study showed that AGO2 was full-bodied in the nucleus of senesced fibroblasts and interacted with miRISC and retinoblastoma (Rb) to suppress the transcription of proliferation-promoting genes regulated by Rb/E2F. Information technology was noted that allow-7f was bound to MREs found in the promoters of ii E2F target genes, CDCA8 and CDC2, in an AGO2-dependent manner (sixty). Besides, a subset of Agone-promoter bound genes was upregulated following senescence, and AGO2 was found to co-immunoprecipitate with euchromatin (sixty). A more recent study by Miao et al. (66) found nuclear miR-522 interacting with a DNA cruciform construction (a stem-loop on sense and antisense DNA strands) inside the promoter of CYP2E1 and suppressing its transcription (66). miRNAs have also been shown to interact at genomic loci, where enhancer-derived RNA (eRNA) are transcribed, and increase mRNA levels of adjacent genes by promoting a transcriptionally agile chromatin state (67) while altering culling splicing profiles (64). The overall function of miRISC in the regulation of chromatin country and construction and transcriptional control remain to be adamant, simply these current data advise a transcription factor-like role. It is too possible that miRISC may exist involved in the establishment of de novo methylation, and past extension, the compactification of chromatin into nuclear compartments, and mediation of genomic remodeling.
Dynamics of miRNA Actions
Studies have revealed that miRNA-mediated gene regulation is dynamic and helps to buffer gene expression to a steady state. Information technology is only recently that a more comprehensive agreement of miRNA dynamics has begun to shed light on the highly robust nature of miRNA-mediated factor regulation. Factors that may contribute to the robustness of miRNA-mediated gene regulation include the functionalized compartmentalization and shuttling of miRISC within the cells. The availability and abundancy of miRNAs and their target mRNAs are besides contributing factors in determining which genes are regulated. Although this is not always the instance, miRNA suppression of mRNA targets is not ubiquitous between cell types. Alternative splicing and alternative polyadenylation affecting 3′ UTRs, and cell type-specific RNA bounden proteins that impact target mRNA secondary structures, change the available puddle of MREs (63, 68, 69). This renders subsets of mRNAs sensitive or insensitive to miRNA-mediated gene regulation in a cell type/land-specific fashion.
Subcellular Compartmentalization of miRNAs
miRISC and target mRNA accept been observed to localize in multiple subcellular compartments including rough endoplasmic reticulum (rER) (70), processing (P)-bodies (71), stress granules (SG) (72), the trans-Golgi network (TGN), early/late endosomes (73), multivesicular bodies (MVB) (74), lysosomes (74), mitochondria (75, 76), and the nucleus (66, 77) (Figure two). With regards to miRNA activeness, the result is that components that facilitate miRNA mail service-transcriptional factor regulation are enriched at sites where miRISC:mRNA complexes localize. This in issue acts to spatially enrich mRNA and miRISC concentrations over fourth dimension and promote efficient regulation of cistron expression (70).
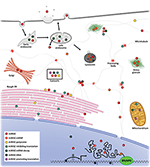
Figure 2. Proposed model of miRNA localization and function. miRISC has been detected in several subcellular locations. In the nucleus, miRISC is enriched at sites of active transcription where information technology can interact with DNA to promote active or inactive chromatin states. Information technology can also interact with nascent mRNA to promote more efficient splicing or alternate splicing profiles. miRISC can interact with nuclear messenger ribonucleoprotein (mRNP) to promote its deposition or remain in A miRISC:mRNP complex equally it is shuttled out of the nucleus. Cytoplasmic miRISC tin lengthened throughout the cytosol or undergo shuttling, well-nigh likely via microtubules. Within the cytosol, miRISC can associate with polysomes, inhibit translation initiation, mediate mRNA disuse, or promote translational activation. On the crude endoplasmic reticulum, miRISC can interact with translating mRNA to inhibit translation. Furthermore, unbound miRISC can also accumulate on the rER to interact with newly rER-leap mRNA. Rough ER miRISC:mRNP complexes that are translationally inhibited can shuttle to the early on/late endosomes to complete mRNA deadenylation and disuse. miRISC can then be recycled into the cytosol or shuttled to the lysosome for degradation. miRISC may likewise localize to transient, membrane-complimentary processing bodies where it can mediate target mRNA translational inhibition and storage or decay. Nether sure cellular weather condition, miRISC:mRNPs may exist shuttled to stress granules for storage and/or deposition. miRISC can also localize to the mitochondria to promote translational activation or mRNA translational inhibition and disuse. Localization of miRISC within the Golgi is likely from vesicles secreted from the early endosome. Moreover, endocytosed miRISC may be shuttled to the Golgi or recycled into the cytosol. Lastly, vesicular or vesicle-gratis miRISC tin exist exocytosed from at least the late endosome into the extracellular milieu to mediate prison cell-cell communication.
P-bodies were identified early on every bit possible sites involved with miRNA-mediated suppressive activity (78). They are cytoplasmic foci depleted of ribosomes (79) and enriched with enzymatic mRNA degradation mechanism, such as mRNA decapping proteins, the CCR4-NOT complex, XRN1, and GW182 family proteins (78). Furthermore, P-bodies grade in an RNA-dependent style as a consequence of RNAi and RNA degradation (80). Recently, P-bodies with agile miRISC have been associated with SYNE1 (nesprin-ane) which acts to attach Argonaute and other P-body components to microtubules (81). Disruption of nesprin-ane action through mutations and RNAi significantly decreased miRNA suppression of target mRNA and P-body germination. On the other manus, stabilization of microtubules with Taxol led to translation inhibition but not mRNA decay of HIF1A following accumulation in P-bodies (82). Importantly, P-body accumulation was miRISC-dependent and reversible as recovery subsequently Taxol handling led to P-torso dissociation dorsum to control levels and HIF1A polysome re-association. It is non clear why a microtubule stabilization would lead to an increment in P-body count. The reduced polysome occupancy of HIF1A observed past Carbonaro et al. (82) may lead to a buildup of HIF1A mRNA on the static microtubule network promoting an increase in HIF1A-bound miRISC. This in plough may enrich miRISC on the static microtubule network and nucleate P-body formation. Typical microtubule instability may in effect dilute microtubule-associated miRISC by preventing enrichment at sites of nucleation. However, the degree to which P-bodies are necessary for efficient miRNA-mediated suppression is uncertain as mRNA degradation machinery exists diffusely throughout the cytoplasm, and at other subcellular locations, and tin can confer RNAi activities even in the absence of P-bodies (78).
Polysomes, which are complexes of mRNA with multiple translating ribosomes, are generally found freely within the cytoplasm or leap to the cytoskeleton or membranous subcellular organelles, such equally rER. miRISC has also been plant to copurify with polysomes, and the miRISC:mRNA complexes bound to these polysomes were associated with increased levels of translational inhibition and mRNA degradation (83). A contempo study showed that miRNAs, AGO2, and target mRNAs are associated with rER-membrane-bound polysomes, and this occurs before miRNA-mediated translational repression (70). Further work by the aforementioned group has led to the finding that rER-bound mRNA, destined for miRNA-dependent deposition, complete this process inside endosomes and MVBs followed by miRISC recycling dorsum into the cytoplasm (73). Importantly, this interaction is miRNA-dependent; disruption of translation, micrococcal nuclease degradation of cytoplasmic mRNA, or mRNA mutants defective cognate MRE led to a loss of polysome/miRISC copurification (70, 84). Additionally, the degree of polysome occupancy of target mRNAs is dependent on the analogousness between miRNA seed region and MRE, merely not on miRNA abundance (84). Moreover, at any given time the average proportion of polysome-bound and -unbound miRISC, chosen the polysome occupancy, was below 40%. These data are in agreement with loftier rates of miRNA-bound AGO sampling of targets observed in vitro (85), i.e., miRISC is able to probe for MREs quickly, just does not form stable interactions on its own. Together these data elude to a key aspect of miRNA functioning; at that place is a crucial equilibrium betwixt MRE bound and unbound states and this equilibrium is important for the spatiotemporal dynamics of miRISC. This allows for miRNA to respond apace to changes in subcellular environments and dynamically regulate many target mRNAs.
A Global View of miRNA at the Cellular Level
The interplay between miRNA abundance/localization, jail cell blazon, cell country, and miRNA-mediated regulation is still nether intense investigation. Recently, the work by the Functional Annotation of the Mammalian Genome (FANTOM5) consortium found that for whatever given human cell blazon, the height v expressed miRNAs represent on average ~50% of the overall miRNA pool (13). Moreover, approximately one-half of the expressed miRNAs are prison cell type enriched, ane quarter are broadly expressed, and the remaining had low-level expression regardless of cell type. These information help fortify the full general roles that miRNA play within cells. By and large, miRNA can regulate cistron networks dynamically and/or transiently, e.g., feedback and feedforward loops (86), and steady state gene regulation, east.one thousand., upregulation of a miRNA throughout differentiation (87). In both cases, college-club effects on gene regulatory networks tin can propagate (88), such as regulation of transcription cistron expression leading to changes in transcriptional profiles. Intriguingly, miRNA practice non solely, or maybe fifty-fifty predominantly, function as target-specific regulators only may play key roles in the post-transcriptional reduction of expression noise (89, 90). In this way miRNA promote stable gene expression past buffering out stochastic fluctuations in transcription. A mutual indicator of expression noise control is high mRNA to poly peptide ratios, rendering reduced translational rates resistant to random fluctuations in mRNA concentration (91), i.east., a small-scale modify to a highly arable mRNA has little effect on protein output. This behavior is seen throughout the brute and bacterial kingdoms, selecting for controlled protein output rather than coupling of mRNA/poly peptide levels (92). Accordingly, the strongest predictor of protein level for any given gene is the rate of translation, followed by mRNA levels (93).
An important concept in connecting miRNA dynamics and cellular cistron expression networks is the idea of MRE load (94). There is an MRE load for each miRNA which represents the total number of available binding sites on all targetable RNA molecules inside a cell. Just put, a miRNA may interact with or sample all available MREs, only the retention time will be longer for higher affinity MREs compared to lower analogousness MREs (94). This has the issue of diluting cellular miRNAs amongst many potential targets such that only a modest proportion of each target mRNA is spring to a cognate miRNA at any given time (94). On the other hand, mRNAs with higher affinity MREs generally show greater sensitivity to miRNA-mediated post-transcriptional repression (84, 94). Yet, the residuum between MRE load and cognate miRNA abundance can lead to varying effects. Increasing the MRE load of a single miRNA, past upregulating i gene, has the effect of sequestering that miRNA away from other targets (94, 95). In this way, modulation of MRE load with competing endogenous RNAs (ceRNAs), such every bit with round RNAs (circRNAs) (96), can act to sequester miRISC from target mRNA, leading to derepression of target mRNA (97). To complicate matters further, by altering subcellular localizations of miRISC, the MRE load can be modulated. At sites of synaptic formation in neurons, rapid and highly specific RNAi takes identify due to the colocalization of target mRNAs and cognate miRNAs (98).
An individual mRNA may contain many MREs (99), and thus contributes to multiple MRE loads for different miRNA. Although to some caste, each private mRNA with multiple MREs may act every bit a ceRNA, multiple MREs can also act to increment miRISC occupancy. Cooperation of multiple, proximal MREs on target mRNA has been shown to increase RNAi activeness and increase retention times of miRISC complexes on those mRNA, nigh likely through the iii Ago bounden sites on the GW182 family proteins (94, 100). In this way, low expression only functional miRNA may synergistically increment RNAi activity on a subset of target mRNAs, thereby effectively enhancing the concentration of cognate miRNAs for specific mRNAs (101, 102). Inversely, low miRNA levels can be compensated for, when target mRNA levels are high, by stimulating increased loading of cognate miRNA into Agone, thereby improving RNAi activity (103). Synergistic furnishings of miRNAs have been shown to be important for biological processes, such as neurogenesis (104) and human embryonic stem cell pluripotency and differentiation (105). Highly synergistic miRNA regulation of target mRNA may also accept implications in diseases, such as oncogenesis. For instance, cyclin-dependent kinase inhibitor 1A (CDKN1A), a tumor suppressor that is downregulated in multiple cancers (106), is targeted by at least 28 miRNAs and many of which are upregulated together in cancers where CDKN1A has been implicated (107). This may suggest that the upregulation of sets of miRNAs in these cancers can synergistically downregulate CDKN1A. miRNA synergism has too been exploited as a therapeutic strategy (108).
In addition to specific miRNA/mRNA dynamics, changes in global miRISC localization in response to changes in cellular environments, such as stress or serum starvation (109), also impact miRNA action. In a study by Wang et al. (110), upon serum starvation of multiple human cell lines, there was an immediate exportation of stable, vesicular or vesicle-free miRNA and a concomitant subtract of intracellular miRNA levels (110). Both cell stress induced by heat shock and translation inhibition following treatment with hippuristanol or cycloheximide induced translocation of miRISC from the nucleus and cytoplasm to transient, cytoplasmic SG (72, 111). SG are known to act as intermediate storage of messenger ribonucleoproteins (mRNPs) that have stalled during translation or following viral infection (112). SG components can also return to the cytoplasm, exchange with P-bodies, or exist digested by lysosomes (81, 112). miRISC that is localized to SG following prison cell stress also contained target mRNAs, leading to a reduction of RNAi activity. The extracellular release of miRNA upon jail cell stress and localization of miRNA:mRNA complexes to SG suggests an mRNA-protective role.
Circulation of miRNAs
Numerous studies have demonstrated that miRNAs can exist released into extracellular fluids. Extracellular miRNAs tin be used as biomarkers for a multifariousness of diseases. These studies have been extensively reviewed (113–115) and therefore volition not be discussed here. The extracellular miRNAs can exist delivered to target cells and they may act as autocrine, paracrine, and/or endocrine regulators to attune cellular activities (116). In this regard, miRNAs have hormone-like activities.
MicroRNAs in Biological Fluids
Many studies take detected extracellular/circulating miRNAs in biological fluids, such as plasma and serum (117, 118), cerebrospinal fluid (119), saliva (120), breast milk (121), urine, tears, colostrum, peritoneal fluid, bronchial lavage, seminal fluid (122), and ovarian follicular fluid (123). Contrary to cellular RNA species, extracellular miRNAs are highly stable, resisting degradation at room temperature for upward to 4 days and in deleterious conditions such as boiling, multiple freeze-thaw cycles, and high or low pH (117, 124).
Two populations of extracellular miRNAs exist in biological fluids. One can be found in vesicles such as exosomes, microvesicles, and apoptotic bodies (116, 120) while the other is associated with proteins, especially AGO2 (120, 125). There accept been some discrepancies on the relative abundancies of these 2 populations. While several studies found that the majority of extracellular miRNAs are not associated with exosomes/microvesicles but are instead bound to AGO2 (118, 125), some other study reported that extracellular miRNAs are present predominantly in exosomes in human serum and saliva (120). Since these studies simply measured a selected group of miRNAs in a few plasma samples, it is possible that the existence of predominantly exosomal or vesicle-costless miRNAs is dependent on the miRNA itself, the cell type they originate from, and/or other factors affecting the secretion of miRNAs in individuals. Other proteins plant to bind extracellular miRNAs include high-density lipoprotein (HDL) (126, 127) and nucleophosmin 1 (NPM1) (110, 118, 128). The presence of miRNAs in vesicles or with accompanying proteins is generally thought to protect extracellular miRNAs and increase their stability in the extracellular milieu (120).
Secretion and Uptake of microRNAs
Although some extracellular miRNAs are regarded as by-products of cellular activities, such as jail cell injury or death (128), increasing evidence suggests that the release of extracellular miRNAs is a regulated procedure. Information technology has been shown that the secretion of exosomal miRNAs is mediated by a ceramide-dependent pathway and the secreted miRNAs exert growth regulatory effects in target cells (129). Recently, it was demonstrated that atheroprotective laminar shear stress induced the release of vesicle-gratuitous miR-126-3p and other miRNAs, as well as AGO2, from endothelial cells by activating vesicle-associated membrane protein iii (VAMP3) and synaptosomal-associated protein 23 (SNAP23) (130). This study also showed that miRNAs secreted from endothelium can regulate the activity of smooth muscle cells (130). In neuroendocrine cells, miRNAs in large dense-core vesicles (LDCVs) are released by exocytosis through vesicle fusion, and this process is mediated past the SNARE complex and accelerated past Ca2+ stimulus (131). Secretion of miRNAs via exosomes have likewise been reported to be regulated by signaling molecules, such as interleukin-4 (IL4) (132) and Docosahexaenoic acrid (DHA) (133). IL4-activated macrophages were found to secrete exosomes conveying oncogenic miRNAs to promote invasiveness of chest cancer cells (132). On the other paw, DHA, which has anticancer and anti-angiogenic activities, induced the secretion of miRNA-containing exosomes that exert inhibitory effects on tumor angiogenesis (133).
Many studies have also demonstrated that extracellular miRNAs tin exert biological functions in recipient cells to regulate their activeness, thereby acting as intercellular signaling molecules. For case, exosome mediated transfer of miR-105 from metastatic chest cancer cells to endothelial cells directly targeted a tight junction protein, zonula occludens 1 (ZO-ane), and this led to the destruction of the barrier office of endothelium and promoted metastasis (134). Moreover, exosomes from umbilical cord blood were plant to be enriched in miR-21-3p, which promoted the proliferation and migration of fibroblasts, and induced the angiogenic activities of endothelial cells, leading to accelerated wound healing (135). miRNAs, specifically miR-342–3p and miR-1246, secreted from a highly metastatic human oral cancer cell line, were found to induce metastasis in a poorly metastatic cancer cell line (136). Extracellular miRNAs accept also been reported to bind to Toll-like receptors (137), activate downstream signaling events, and eventually lead to biological responses, such as tumor growth and metastasis (138), and neurodegeneration (139). Thus, miRNAs may act as chemic messengers to regulate cell-cell communications.
The mechanisms of extracellular miRNA uptake are non well understood. Information technology has been proposed that vesicle-associated extracellular miRNAs may enter cells by endocytosis, phagocytosis, or direct fusion with the plasma membranes, while vesicle-free secreted miRNAs may be taken upwardly past specific receptors on the prison cell surface (140). Indeed, several studies have shown that miRNAs enter recipient cells by endocytosis and micropinocytosis (141, 142). This process has been reported to be dependent on clathrin, merely not on caveolae or lipid rafts in PC12 cells (142). However, some other study conducted in A549-P cells showed that endocytosis of exosomal miRNAs is mediated by caveolae- and lipid raft-dependent pathways (143). Furthermore, vesicle-free miRNAs associated with HDL are taken up by HDL receptor and scavenger receptor BI (SR-BI) receptor in the plasma membrane of the target cells (126, 127). miRNAs have also been shown to transfer between co-cultured cells via direct prison cell-jail cell contact and gap junctions (144). While these studies suggest that extracellular miRNAs tin collaborate with recipient cells via multiple mechanisms, the factors that decide the specificity of such interactions demand to be investigated.
Concluding Remarks
Since the discovery of miRNAs in the earlier 1990s, tremendous progress has been made on how miRNAs are produced within cells, how they exert regulatory furnishings on gene expression, and how they are involved in various physiological and pathological events. It is now clear that miRNAs are powerful gene regulators, and that they not but help control mRNA stability and translation merely are besides involved in transcription. Yet, our understanding of when and how miRNAs can exert regulatory effects on transcription is express. Similarly, the weather under which miRNAs elicit translational activation need to be further explored. In improver, careful analysis and consideration of experimental techniques and model systems should exist employed when attempting to generalize miRNA capabilities. The assaying of miRNA action inside a exam tube may not be recapitulated inside the cellular environment and thus should be viewed with circumspection. Many studies take been conducted in vitro past transfecting pre-miRNAs or mature mRNA mimics into immortalized and cancer prison cell lines. The extent to which findings from such studies reverberate the endogenous miRNA functions in vivo requires further study. Also, the add-on of chemical tags to miRNA could also impact miRNA:MRE interaction or AGO:miRNA interactions, specifically with v′ and 3′ miRNA nucleotide modifications. The first and final miRNA nucleotides clamp the miRNA within Agone proteins, and thus tags to these regions may affect miRNA operation in unpredictable ways.
Recent studies have shed lite on the dynamic nature of miRNA actions and further revealed the complexity of miRNA-mediated gene regulation. Many factors contribute to the activity of miRNAs, including subcellular location, miRNA/mRNA abundance, miRNA:MRE affinity, cell blazon/land, and the availability of various miRISC components. miRNAs in the nucleus play a office in regulating transcription and culling splicing. Cytosolic miRISC components shuttle between different compartments. Moreover, their localization, together with the levels of miRNAs and target mRNAs, and the affinity of the miRNA-mRNA interaction, are important for efficient factor regulation. Recent advances in single molecule imaging will greatly impact the field, as has already begun. Viewing the move of single miRNA and/or mRNA with high spatial and temporal resolution will aid u.s.a. sympathise this complex dynamic in an unprecedented manner. Investigation of large scale, global miRNA interactomes will also propel the field forward, allowing powerful mathematical models to exist applied to highly complex regulatory networks.
It is now accepted that extracellular/circulating miRNAs can not simply serve as biomarkers for diseases, but also play of import roles in intercellular communication. miRNAs regulate the activity of host cells, and they are besides secreted and transferred to recipient cells. Many studies have shown that extracellular miRNAs are functionally active in recipient cells. Some miRNAs can even interact with prison cell surface receptors, such equally Toll-like receptors. Therefore, miRNAs have hormone-similar activities. However, well-nigh studies conducted so far were washed in vitro using co-culture of unlike cell types. More in vivo studies are required to make up one's mind whether miRNAs target specific cells under physiological weather condition. Although miRceptors have been proposed (137), apart from the Price-like receptors, they remain to be identified. Mechanisms by which miRNAs are secreted and taken upwards by cells are non well understood and require further investigation.
Author Contributions
All authors listed have made a substantial, directly and intellectual contribution to the work, and approved it for publication.
Funding
Work in our laboratory was supported by grants from the Canadian Institutes of Wellness Research (MOP-81370, CCI-92222, CCI-132565, MOP-89931, and PJT-153146) and Natural Science and Technology Inquiry Council to CP. JO and HH were supported by the Ontario Graduate Scholarship.
Conflict of Interest Statement
The authors declare that the enquiry was conducted in the absence of whatsoever commercial or financial relationships that could be construed as a potential conflict of interest.
References
one. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-iv encodes small RNAs with antisense complementarity to lin-14. Cell (1993) 75:843–54. doi: 10.1016/0092-8674(93)90529-Y
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Wightman B, Ha I, Ruvkun K. Posttranscriptional regulation of the heterochronic factor lin-14 by lin-four mediates temporal pattern germination in C. elegans. Jail cell (1993) 75:855–62. doi: 10.1016/0092-8674(93)90530-4
PubMed Abstract | CrossRef Total Text | Google Scholar
3. Horvitz HR, Sulston JE. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics (1980) 96:435–54.
PubMed Abstract | Google Scholar
v. Ambros Five, Horvitz Hour. The lin-xiv locus of Caenorhabditis elegans controls the time of expression of specific postembryonic developmental events. Genes Dev. (1987) 1:398–414. doi: 10.1101/gad.1.4.398
PubMed Abstruse | CrossRef Full Text | Google Scholar
6. Ferguson EL, Sternberg Prisoner of war, Horvitz HR. A genetic pathway for the specification of the vulval cell lineages of Caenorhabditis elegans. Nature (1987) 326:259–67.
PubMed Abstract | Google Scholar
9. Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-vii heterochronic regulatory RNA. Nature (2000) 408:86–ix. doi: 10.1038/35040556
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Friedlander MR, Lizano E, Houben AJ, Bezdan D, Banez-Coronel 1000, Kudla Grand, et al. Show for the biogenesis of more i,000 novel homo microRNAs. Genome Biol. (2014) 15:R57. doi: 10.1186/gb-2014-15-four-r57
PubMed Abstruse | CrossRef Full Text | Google Scholar
13. de Rie D, Abugessaisa I, Alam T, Arner Due east, Arner P, Ashoor H, et al. An integrated expression atlas of miRNAs and their promoters in man and mouse. Nat Biotechnol. (2017) 35:872–8. doi: x.1038/nbt.3947
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol Cell. (2016) 64:320–33. doi: ten.1016/j.molcel.2016.09.004
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Makarova JA, Shkurnikov MU, Wicklein D, Lange T, Samatov TR, Turchinovich AA, et al. Intracellular and extracellular microRNA: an update on localization and biological role. Prog Histochem Cytochem. (2016) 51:33–49. doi: 10.1016/j.proghi.2016.06.001
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Okada C, Yamashita E, Lee SJ, Shibata Due south, Katahira J, Nakagawa A, et al. A high-resolution construction of the pre-microRNA nuclear export machinery. Science (2009) 326:1275–9. doi: ten.1126/science.1178705
PubMed Abstract | CrossRef Total Text | Google Scholar
31. Zhang H, Kolb FA, Jaskiewicz L, Westhof East, Filipowicz W. Unmarried processing eye models for homo Dicer and bacterial RNase III. Cell (2004) 118:57–68. doi: 10.1016/j.cell.2004.06.017
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Babiarz JE, Scarlet JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. (2008) 22:2773–85. doi: 10.1101/gad.1705308
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Xie M, Li Chiliad, Vilborg A, Lee North, Shu Medico, Yartseva V, et al. Mammalian 5′-capped microRNA precursors that generate a single microRNA. Jail cell (2013) 155:1568–fourscore. doi: 10.1016/j.cell.2013.11.027
PubMed Abstruse | CrossRef Full Text | Google Scholar
38. Yang JS, Maurin T, Robine N, Rasmussen KD, Jeffrey KL, Chandwani R, et al. Conserved vertebrate mir-451 provides a platform for Dicer-independent, Ago2-mediated microRNA biogenesis. Proc Natl Acad Sci United states of america. (2010) 107:15163–viii. doi: 10.1073/pnas.1006432107
PubMed Abstract | CrossRef Full Text | Google Scholar
43. Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the permit-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci The states. (2008) 105:14879–84. doi: 10.1073/pnas.0803230105
PubMed Abstract | CrossRef Total Text | Google Scholar
44. Zhang J, Zhou Due west, Liu Y, Liu T, Li C, Wang L. Oncogenic role of microRNA-532-5p in human colorectal cancer via targeting of the 5′ UTR of RUNX3. Oncol Lett. (2018) 15:7215–xx. doi: x.3892/ol.2018.8217
CrossRef Full Text | Google Scholar
45. Dharap A, Pokrzywa C, Murali Southward, Pandi G, Vemuganti R. MicroRNA miR-324-3p induces promoter-mediated expression of RelA factor. PLoS ONE (2013) eight:e79467. doi: 10.1371/journal.pone.0079467
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Ameres SL, Horwich Physician, Hung JH, Xu J, Ghildiyal 1000, Weng Z, et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science (2010) 328:1534–9. doi: 10.1126/scientific discipline.1187058
PubMed Abstract | CrossRef Full Text | Google Scholar
52. Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde Due east. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. (2006) twenty:1885–98. doi: x.1101/gad.1424106
PubMed Abstract | CrossRef Full Text | Google Scholar
53. Christie M, Boland A, Huntzinger E, Weichenrieder O, Izaurralde E. Structure of the PAN3 pseudokinase reveals the basis for interactions with the PAN2 deadenylase and the GW182 proteins. Mol Cell. (2013) 51:360–73. doi: x.1016/j.molcel.2013.07.011
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Braun JE, Truffault V, Boland A, Huntzinger East, Chang CT, Haas G, et al. A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat Struct Mol Biol. (2012) 19:1324–31. doi: 10.1038/nsmb.2413
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Truesdell SS, Mortensen RD, Seo M, Schroeder JC, Lee JH, LeTonqueze O, et al. MicroRNA-mediated mRNA translation activation in quiescent cells and oocytes involves recruitment of a nuclear microRNP. Sci Rep. (2012) 2:842. doi: 10.1038/srep00842
PubMed Abstruse | CrossRef Total Text | Google Scholar
57. Bukhari SIA, Truesdell SS, Lee S, Kollu South, Classon A, Boukhali M, et al. A specialized machinery of translation mediated by FXR1a-associated microRNP in cellular quiescence. Mol Prison cell. (2016) 61:760–73. doi: ten.1016/j.molcel.2016.02.013
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Prison cell. (2008) xxx:460–71. doi: ten.1016/j.molcel.2008.05.001
PubMed Abstract | CrossRef Full Text | Google Scholar
59. Nishi One thousand, Nishi A, Nagasawa T, Ui-Tei One thousand. Human being TNRC6A is an Argonaute-navigator protein for microRNA-mediated factor silencing in the nucleus. RNA (2013) nineteen:17–35. doi: 10.1261/rna.034769.112
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Benhamed M, Herbig U, Ye T, Dejean A, Bischof O. Senescence is an endogenous trigger for microRNA-directed transcriptional factor silencing in human cells. Nat Prison cell Biol. (2012) 14:266–75. doi: ten.1038/ncb2443
PubMed Abstruse | CrossRef Total Text | Google Scholar
61. Pitchiaya S, Heinicke LA, Park JI, Cameron EL, Walter NG. Resolving subcellular miRNA trafficking and turnover at single-molecule resolution. Cell Rep. (2017) 19:630–42. doi: x.1016/j.celrep.2017.03.075
PubMed Abstract | CrossRef Full Text | Google Scholar
62. Cernilogar FM, Onorati MC, Kothe Get, Burroughs AM, Parsi KM, Breiling A, et al. Chromatin-associated RNA interference components contribute to transcriptional regulation in Drosophila. Nature (2011) 480:391–5. doi: 10.1038/nature10492
PubMed Abstract | CrossRef Full Text | Google Scholar
63. Bottini S, Hamouda-Tekaya Due north, Mategot R, Zaragosi LE, Audebert South, Pisano Due south, et al. Mail-transcriptional factor silencing mediated by microRNAs is controlled by nucleoplasmic Sfpq. Nat Commun. (2017) 8:1189. doi: 10.1038/s41467-017-01126-x
PubMed Abstruse | CrossRef Full Text | Google Scholar
64. Allo M, Agirre Due east, Bessonov S, Bertucci P, Gomez Acuna L, Buggiano 5, et al. Argonaute-1 binds transcriptional enhancers and controls constitutive and culling splicing in human cells. Proc Natl Acad Sci Usa. (2014) 111:15622–9. doi: 10.1073/pnas.1416858111
PubMed Abstract | CrossRef Full Text | Google Scholar
66. Miao L, Yao H, Li C, Pu K, Yao 10, Yang H, et al. A dual inhibition: microRNA-552 suppresses both transcription and translation of cytochrome P450 2E1. Biochim Biophys Acta (2016) 1859:650–62. doi: x.1016/j.bbagrm.2016.02.016
PubMed Abstract | CrossRef Full Text | Google Scholar
67. Xiao Thousand, Li J, Li West, Wang Y, Wu F, Xi Y, et al. MicroRNAs actuate gene transcription epigenetically as an enhancer trigger. RNA Biol. (2017) 14:1326–34. doi: x.1080/15476286.2015.1112487
PubMed Abstruse | CrossRef Full Text | Google Scholar
68. Nam JW, Rissland Os, Koppstein D, Abreu-Goodger C, Jan CH, Agarwal V, et al. Global analyses of the consequence of different cellular contexts on microRNA targeting. Mol Prison cell. (2014) 53:1031–43. doi: x.1016/j.molcel.2014.02.013
PubMed Abstract | CrossRef Full Text | Google Scholar
69. Blazie SM, Geissel HC, Wilky H, Joshi R, Newbern J, Mangone M. Alternative polyadenylation directs tissue-specific miRNA targeting in Caenorhabditis elegans Somatic Tissues. Genetics (2017) 206:757–74. doi: x.1534/genetics.116.196774
PubMed Abstract | CrossRef Full Text | Google Scholar
70. Barman B, Bhattacharyya SN. mRNA targeting to endoplasmic reticulum precedes ago poly peptide interaction and microRNA (miRNA)-mediated translation repression in mammalian cells. J Biol Chem. (2015) 290:24650–6. doi: 10.1074/jbc.C115.661868
PubMed Abstract | CrossRef Full Text | Google Scholar
71. Nishi Thousand, Takahashi T, Suzawa Thousand, Miyakawa T, Nagasawa T, Ming Y, et al. Command of the localization and function of a miRNA silencing component TNRC6A past Argonaute poly peptide. Nucleic Acids Res (2015) 43:9856–73. doi: 10.1093/nar/gkv1026
PubMed Abstract | CrossRef Full Text | Google Scholar
72. Detzer A, Engel C, Wunsche W, Sczakiel G. Cell stress is related to re-localization of Argonaute 2 and to decreased RNA interference in human being cells. Nucleic Acids Res. (2011) 39:2727–41. doi: 10.1093/nar/gkq1216
PubMed Abstruse | CrossRef Total Text | Google Scholar
73. Bose M, Barman B, Goswami A, Bhattacharyya SN. Spatiotemporal uncoupling of MicroRNA-mediated translational repression and target RNA degradation controls microRNP recycling in mammalian cells. Mol Jail cell Biol. (2017) 37:e00464–xvi. doi: ten.1128/MCB.00464-16
PubMed Abstruse | CrossRef Full Text | Google Scholar
74. Gibbings DJ, Ciaudo C, Erhardt Chiliad, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and attune miRNA activity. Nat Cell Biol. (2009) 11:1143–9. doi: x.1038/ncb1929
PubMed Abstract | CrossRef Full Text | Google Scholar
75. Barrey E, Saint-Auret G, Bonnamy B, Damas D, Boyer O, Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS One (2011) 6:e20220. doi: 10.1371/journal.pone.0020220
PubMed Abstruse | CrossRef Full Text | Google Scholar
76. Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell (2014) 158:607–xix. doi: 10.1016/j.cell.2014.05.047
PubMed Abstruse | CrossRef Full Text | Google Scholar
80. Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde Eastward. P-torso formation is a event, not the cause, of RNA-mediated gene silencing. Mol Cell Biol. (2007) 27:3970–81. doi: 10.1128/MCB.00128-07
CrossRef Full Text | Google Scholar
81. Rajgor D, Mellad JA, Soong D, Rattner JB, Fritzler MJ, Shanahan CM. Mammalian microtubule P-trunk dynamics are mediated by nesprin-1. J Prison cell Biol. (2014) 205:457–75. doi: 10.1083/jcb.201306076
PubMed Abstract | CrossRef Total Text | Google Scholar
82. Carbonaro M, O'Brate A, Giannakakou P. Microtubule disruption targets HIF-1alpha mRNA to cytoplasmic P-bodies for translational repression. J Jail cell Biol. (2011) 192:83–99. doi: 10.1083/jcb.201004145
PubMed Abstruse | CrossRef Full Text | Google Scholar
83. Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot Due north, Basyuk E, et al. Inhibition of translational initiation by Let-seven MicroRNA in human being cells. Science (2005) 309:1573–6. doi: 10.1126/science.1115079
PubMed Abstruse | CrossRef Full Text | Google Scholar
84. Molotski Northward, Soen Y. Differential association of microRNAs with polysomes reflects distinct strengths of interactions with their mRNA targets. RNA (2012) 18:1612–23. doi: 10.1261/rna.033142.112
PubMed Abstract | CrossRef Total Text | Google Scholar
85. Wee LM, Flores-Jasso CF, Salomon Nosotros, Zamore PD. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Prison cell (2012) 151:1055–67. doi: 10.1016/j.cell.2012.10.036
PubMed Abstruse | CrossRef Full Text | Google Scholar
86. Lai 10, Wolkenhauer O, Vera J. Agreement microRNA-mediated gene regulatory networks through mathematical modelling. Nucleic Acids Res. (2016) 44:6019–35. doi: 10.1093/nar/gkw550
PubMed Abstruse | CrossRef Full Text | Google Scholar
87. Zhang JF, Fu WM, He ML, Xie WD, Lv Q, Wan One thousand, et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. (2011) 8:829–38. doi: 10.4161/rna.viii.5.16043
PubMed Abstract | CrossRef Total Text | Google Scholar
89. Siciliano 5, Garzilli I, Fracassi C, Criscuolo S, Ventre Due south, di Bernardo D. MiRNAs confer phenotypic robustness to gene networks by suppressing biological racket. Nat Commun. (2013) 4:2364. doi: ten.1038/ncomms3364
PubMed Abstract | CrossRef Full Text | Google Scholar
92. Lalanne JB, Taggart JC, Guo MS, Herzel L, Schieler A, Li GW. Evolutionary convergence of pathway-specific enzyme expression stoichiometry. Cell (2018) 173:749–761. doi: 10.1016/j.prison cell.2018.03.007
PubMed Abstract | CrossRef Full Text | Google Scholar
93. Schwanhäusser B, Busse D, Li North, Dittmar M, Schuchhardt J, Wolf J, et al. Global quantification of mammalian cistron expression command. Nature (2011) 473:337–42. doi: 10.1038/nature10098
PubMed Abstract | CrossRef Total Text | Google Scholar
94. Denzler R, McGeary SE, Title AC, Agarwal V, Bartel DP, Stoffel Thousand. Bear on of MicroRNA levels, target-site complementarity, and cooperativity on competing endogenous RNA-regulated gene expression. Mol Prison cell (2016) 64:565–79. doi: x.1016/j.molcel.2016.09.027
PubMed Abstract | CrossRef Full Text | Google Scholar
97. Yu CY, Li TC, Wu YY, Yeh CH, Chiang W, Chuang CY, et al. The round RNA circBIRC6 participates in the molecular circuitry decision-making human pluripotency. Nat Commun. (2017) eight:1149. doi: ten.1038/s41467-017-01216-w
PubMed Abstruse | CrossRef Total Text | Google Scholar
98. Sambandan Southward, Akbalik G, Kochen L, Rinne J, Kahlstatt J, Glock C, et al. Activity-dependent spatially localized miRNA maturation in neuronal dendrites. Scientific discipline (2017) 355:634–7. doi: 10.1126/science.aaf8995
PubMed Abstract | CrossRef Total Text | Google Scholar
99. Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. (2018) 46:D296–D302. doi: x.1093/nar/gkx1067
PubMed Abstract | CrossRef Full Text | Google Scholar
100. Elkayam Due east, Faehnle CR, Morales G, Sun J, Li H, Joshua-Tor L. Multivalent recruitment of homo argonaute by GW182. Mol Cell. (2017) 67:646–658.e3. doi: 10.1016/j.molcel.2017.07.007
PubMed Abstract | CrossRef Full Text | Google Scholar
101. Li Y, Liang C, Wong KC, Luo J, Zhang Z. Mirsynergy: detecting synergistic miRNA regulatory modules by overlapping neighbourhood expansion. Bioinformatics (2014) thirty:2627–35. doi: x.1093/bioinformatics/btu373
PubMed Abstract | CrossRef Full Text | Google Scholar
102. Lemus-Diaz N, Boker KO, Rodriguez-Polo I, Mitter M, Preis J, Arlt M, et al. Dissecting miRNA gene repression on unmarried cell level with an advanced fluorescent reporter system. Sci Rep. (2017) seven:45197. doi: 10.1038/srep45197
PubMed Abstract | CrossRef Full Text | Google Scholar
104. Pons-Espinal M, de Luca E, Marzi MJ, Beckervordersandforth R, Armirotti A, Nicassio F, et al. Synergic functions of miRNAs determine neuronal fate of adult neural stem cells. Stalk Cell Rep. (2017) 8:1046–61. doi: 10.1016/j.stemcr.2017.02.012
PubMed Abstract | CrossRef Full Text | Google Scholar
105. Sahu M, Mallick B. Deciphering synergistic regulatory networks of microRNAs in hESCs and fibroblasts. Int J Biol Macromol. (2018) 113:1279–86. doi: 10.1016/j.ijbiomac.2018.03.061
PubMed Abstract | CrossRef Full Text | Google Scholar
107. Wu Southward, Huang S, Ding J, Zhao Y, Liang L, Liu T, et al. Multiple microRNAs attune p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene (2010) 29:2302–eight. doi: 10.1038/onc.2010.34
PubMed Abstract | CrossRef Full Text | Google Scholar
108. Yao W, Guo Thou, Zhang Q, Fan L, Wu N, Bo Y. The application of multiple miRNA response elements enables oncolytic adenoviruses to possess specificity to glioma cells. Virology (2014). 458–459, 69–82. doi: 10.1016/j.virol.2014.04.007
PubMed Abstract | CrossRef Full Text | Google Scholar
109. Kucherenko MM, Shcherbata HR. miRNA targeting and alternative splicing in the stress response - events hosted by membrane-less compartments. J Cell Sci. (2018) 131:jcs202002. doi: x.1242/jcs.202002
PubMed Abstract | CrossRef Full Text | Google Scholar
110. Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Consign of microRNAs and microRNA-protective protein past mammalian cells. Nucleic Acids Res. (2010) 38:7248–59. doi: ten.1093/nar/gkq601
PubMed Abstract | CrossRef Full Text | Google Scholar
111. Leung AK, Calabrese JM, Sharp PA. Quantitative assay of Argonaute poly peptide reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci Usa. (2006) 103:18125–30. doi: ten.1073/pnas.0608845103
PubMed Abstract | CrossRef Full Text | Google Scholar
114. Sohn Westward, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. (2015) 47:e184. doi: ten.1038/emm.2015.68
PubMed Abstract | CrossRef Full Text | Google Scholar
115. Pereira-da-Silva T, Coutinho Cruz Thousand, Carrusca C, Cruz Ferreira R, Napoleao P, Mota Carmo M. Circulating microRNA profiles in different arterial territories of stable atherosclerotic disease: a systematic review. Am J Cardiovasc Dis. (2018) 8:1–thirteen.
PubMed Abstruse | Google Scholar
116. Iftikhar H, Carney GE. Prove and potential in vivo functions for biofluid miRNAs: from expression profiling to functional testing: potential roles of extracellular miRNAs every bit indicators of physiological change and as agents of intercellular information exchange. Bioessays (2016) 38:367–78. doi: 10.1002/bies.201500130
PubMed Abstract | CrossRef Full Text | Google Scholar
117. Chen 10, Ba Y, Ma L, Cai Ten, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel grade of biomarkers for diagnosis of cancer and other diseases. Cell Res (2008) 18:997–1006. doi: 10.1038/cr.2008.282
PubMed Abstract | CrossRef Total Text | Google Scholar
118. Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes bear a population of circulating microRNAs contained of vesicles in human plasma. Proc Natl Acad Sci USA (2011) 108:5003–8. doi: 10.1073/pnas.1019055108
PubMed Abstract | CrossRef Full Text | Google Scholar
119. Cogswell JP, Ward J, Taylor IA, Waters Yard, Shi Y, Cannon B, et al. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into affliction pathways. J Alzheimers Dis. (2008) 14:27–41. doi: 10.3233/JAD-2008-14103
PubMed Abstract | CrossRef Full Text | Google Scholar
120. Gallo A, Tandon M, Alevizos I, Illei GG. The bulk of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS 1 (2012) 7:e30679. doi: 10.1371/journal.pone.0030679
PubMed Abstract | CrossRef Full Text | Google Scholar
123. da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Jail cell-secreted vesicles in equine ovarian follicular fluid incorporate miRNAs and proteins: a possible new class of prison cell communication within the ovarian follicle. Biol Reprod. (2012) 86:71. doi: 10.1095/biolreprod.111.093252
PubMed Abstract | CrossRef Full Text | Google Scholar
124. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. (2008) 105:10513–8. doi: ten.1073/pnas.0804549105
PubMed Abstract | CrossRef Full Text | Google Scholar
126. Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. (2011) xiii:423–33. doi: 10.1038/ncb2210
PubMed Abstruse | CrossRef Total Text | Google Scholar
127. Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, et al. HDL-transferred microRNA-223 regulates ICAM-ane expression in endothelial cells. Nat Commun. (2014) five:3292. doi: x.1038/ncomms4292
PubMed Abstract | CrossRef Full Text | Google Scholar
129. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. (2010) 285:17442–52. doi: 10.1074/jbc.M110.107821
PubMed Abstract | CrossRef Full Text | Google Scholar
130. Zhu JJ, Liu YF, Zhang YP, Zhao CR, Yao WJ, Li YS, et al. VAMP3 and SNAP23 mediate the disturbed flow-induced endothelial microRNA secretion and smooth muscle hyperplasia. Proc Natl Acad Sci USA. (2017) 114:8271–6. doi: 10.1073/pnas.1700561114
PubMed Abstract | CrossRef Full Text | Google Scholar
132. Yang M, Chen J, Su F, Yu B, Su F, Lin L, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer (2011) 10:117. doi: x.1186/1476-4598-10-117
PubMed Abstract | CrossRef Full Text | Google Scholar
133. Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is contradistinct by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer (2015) fourteen:133. doi: x.1186/s12943-015-0400-vii
PubMed Abstract | CrossRef Total Text | Google Scholar
134. Zhou Due west, Fong MY, Min Y, Somlo G, Liu 50, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Prison cell (2014) 25:501–15. doi: 10.1016/j.ccr.2014.03.007
PubMed Abstruse | CrossRef Total Text | Google Scholar
135. Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics (2018) 8:169–84. doi: 10.7150/thno.21234
PubMed Abstruse | CrossRef Total Text | Google Scholar
136. Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell movement and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. (2016) 6:38750. doi: 10.1038/srep38750
PubMed Abstract | CrossRef Full Text | Google Scholar
137. Fabbri M. MicroRNAs and miRceptors: a new mechanism of activeness for intercellular communication. Philos Trans R Soc Lond B Biol Sci. (2018) 373:E2110–half dozen. doi: 10.1098/rstb.2016.0486
PubMed Abstract | CrossRef Full Text | Google Scholar
138. Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Cost-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. (2012) 109:E2110–E2116. doi: 10.1073/pnas.1209414109
PubMed Abstract | CrossRef Total Text | Google Scholar
139. Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, et al. An anarchistic role for miRNA: let-7 activates Toll-similar receptor 7 and causes neurodegeneration. Nat Neurosci. (2012) 15:827–35. doi: 10.1038/nn.3113
PubMed Abstract | CrossRef Total Text | Google Scholar
141. Xu J, Chen Q, Zen 1000, Zhang C, Zhang Q. Synaptosomes secrete and uptake functionally active microRNAs via exocytosis and endocytosis pathways. J Neurochem. (2013) 124:15–25. doi: 10.1111/jnc.12057
PubMed Abstruse | CrossRef Full Text | Google Scholar
142. Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. (2014) 289:22258–67. doi: 10.1074/jbc.M114.588046
PubMed Abstract | CrossRef Full Text | Google Scholar
143. Wei F, Ma C, Zhou T, Dong Ten, Luo Q, Geng L, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via commitment of miRNA-222-3p. Mol Cancer (2017) 16:132. doi: 10.1186/s12943-017-0694-8
PubMed Abstract | CrossRef Full Text | Google Scholar
144. Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. (2013) 191:6250–60. doi: x.4049/jimmunol.1301728
PubMed Abstruse | CrossRef Total Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fendo.2018.00402/full
Post a Comment for "Place the Steps in the Correct Order to Review the Process of Enzyme Repression"